HIV -- AIDS
The human immunodeficiency virus (HIV) is a retrovirus which causes acquired immune deficiency syndrome (AIDS) by its effects on the human immune system. A very small minority of scientists continue to question the connection between HIV and AIDS and even the very existence of HIV (see AIDS reappraisal).
History
The first AIDS cases were described in 1981. HIV was first proposed as the cause of AIDS by Luc Montagnier of France and Robert Gallo of the United States in 1983-1984, leading to some controversy regarding the priority. At the time, the virus was called Human T-Lymphotropic Virus type III (HTLV-III) or Lymphadenopathy-Associated Virus (LAV). In 1986, the genome of the virus was cloned and sequenced. The name HIV has been in use since 1986.
As of the end of 2004, there were an estimated 39.4 million people around the world living with HIV or AIDS, 25.4 million of whom were in sub-Saharan Africa.
In some parts of the United States, it is illegal for a person with HIV to knowingly infect a person with the virus. This is also the case in most Western countries.
At 13/2/2005, news reported that a man in New York is found to be infected with one multi-drug resistant HIV called 3-DCR HIV (3-Drug-Class-Resistant HIV-1). See here (http://www.nyc.gov/html/doh/html/public/press05/pr016-05.html) for more information. It is important to note that this is only one case, and it has been reported in a press release rather than a properly peer-reviewed journal. The press release has few details about the actual case. It is therefore too early to read too much into this reported case.
Signs and symptoms
Acute infection with HIV is a very aspecific syndrome, which is easily missed due to its likeness to infectious mononucleosis and other viral infections. Fever, fatigue and rash are the most common symptoms, and many develop lymphadenopathy (swollen lymph nodes). Pharyngitis, myalgia and several other symptoms also occur (Kahn & Walker, 1998).
This seroconversion syndrome is different from AIDS, the immune disease that most untreated HIV-infected patients develop eventually.
A very small minority of scientists continue to question the connection between HIV and AIDS and even the very existence of HIV (see AIDS reappraisal).
Shortly after infection, the body produces specific antibodies; most HIV tests work by detecting the presence of these antibodies.
The virus
HIV has several major genes coding for structural proteins that are found in all retroviruses, and several non-structural or "accessory" genes that are unique to HIV.
- General retrovirus genes
- gag. gag-derived proteins make up the cone-shaped viral capsid (p24, i.e. a 24 Kilodalton protein, CA) the nucleocapsid proteins (p6 and p7, NC) and a matrix protein (p17, MA).
- pol. The pol gene codes for the virus enzymatically active proteins. Most important is the so-called reverse transcriptase (RT) which performs the unique reverse transcription of the viral RNA into double-stranded DNA. The latter is integrated into the genome of the host, which means into a chromosome of an infected cell of an HIV-positive person by the pol-encode integrase (IN). Also, pol encodes a specific viral protease (PR). This enzyme cleaves gag- and gag-pol-derived proteins into functional pieces.
- env. env stands for "envelope". The proteins derived from env are a surface (gp120) and a transmembrane protein (gp41). They are located at the outer part of the virus particle and enable the virus to attach to and to fuse with the target cells to initiate the infectious cycle. The gene-product has a knoblike structure.
- Specific
HIV genes
- tat. A portion of the HIV RNA structure is a hairpin structure which initially prevents full transcription taking place. Part of the RNA that is transcribed (ie. before the hairpin portion) encodes the tat protein. tat binds to CdK9/CycT and phosphorylates it, helping to alter its shape and eliminating the effect of the hairpin RNA structure. This itself increases the rate of trancription, providing a positive feedback cycle. This allows HIV to have an explosive response once a threshold amount of tat is produced, a useful tool in defeating the body's response.
- rev. rev allows fragments of HIV mRNA that contain a rev Response Unit (RRE) to be exported from the nucleus to the cytoplasm. In the absence of rev, RNA splicing machinery in the nucleus quickly splices the RNA so that only the smaller, regulatory proteins can be produced; in the presence of rev, RNA is exported from the nucleus before it can be spliced so that the structural proteins and RNA genome can be produced. Again, this mechanism allows a positive feedback loop and allows HIV to overwhelm the host's defences, and it also provides time-dependant regulation of replication (a common theme in virus infections).
- nef. nef is involved in how pathogenic the virus it. It downregulates the CD4 molecule on T cells (so they can't respond to infections as well). One group of people in Sydney, Australia were infected with a nef-deleted virus and took much longer than expected to progress to AIDS. Unfortunately they did, and a nef-deleted virus vaccine has failed also (in animals - it never made it to human trials).
- vif. vif helps the virus infect cells after it leaves the one it's in. It appears to be involved in how the RNA genome and Gag protein bind to each other and also inhibits a cellular protein that modifies RNA.
- vpr. vpr is involved in getting the virus into the nucleus of the cell so it can integrate. It also causes the cell to stop growing, which can result in immune dysfunction.
- vpu. vpu is involved in getting the virus out of the cell. It enhances virion release from the cell. In HIV-2 the gene is called vpx.
Types of HIV and their origins
Since 1986, it has been recognized that there are two species of the virus: HIV-1 and HIV-2. They differ in their genome and in their infectivity, with HIV-1 being well over three times as infective as HIV-2 (Gilbert 2002). Furthermore, it takes longer for HIV-2 infection to cause disease. HIV-2 infections are mostly found in Western Africa and, increasingly, in India; HIV-1 is endemic in the rest of the world.
Both HIV types are closely related to Simian Immunodeficiency Virus (SIV), with HIV-1 more closely related to the chimpanzee strain of SIV than to HIV-2, and HIV-2 more closely related to the Sooty Mangabey strain of SIV than to HIV-1. It is likely that HIV was introduced into humans in Africa via contact with the blood of hunted monkeys, in the first half of the 20th century (Sharp 2001).
HIV-1 is further subdivided into three groups: M, O and N. The groups N and O appear to be confined to Africa, with N being very rare. The three groups probably resulted from three different human/monkey contacts. The major group, M, is further divided into numerous subgroups or clades, identified by letters.
Phylogenetic analyses show that the HIV-1 M group ancestral sequence most probably first occurred at some time between 1915 and 1941 (B. Korber et al. "Timing the Ancestor of the HIV-1 Pandemic Strains." Science 288, 9 June 2000 (http://www.cmu.edu/mcs/Merck/Korber.pdf)).
HIV and the immune response
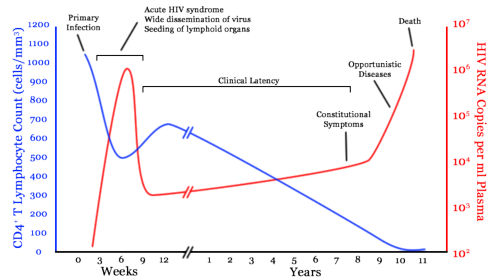
Infection begins with an acute viremia. After this acute phase, the virus count drops up to 100 fold. From this alone, we see that the body seems to have a response to the HIV virus.
After the acute viremia, a period of clinical latency begins. At first this was believed to be true viral latency whereby the HIV was inserted in the host genome in an unproductive state awaiting certain body conditions to begin transcription. This implied the final fatal phase was just a breakdown of the asymptomatic phase causing transcription. There was subsequently a great deal of research into HIV transcription factors. Unfortunately, until about 1993, the sensitivity of viral assays was very poor meaning useful advances were not possible. The use of PCR amplification techniques from 1993 onwards meant that viral counts as low as 50 copies/ml were now detectable.
Around this time, attention also switched to the analysis of HIV in lymphoid tissue. Dendritic cells were found coated with virions, showing that the so called latent phase is not latent at all, virus levels are still high.
Steady-state hypothesis
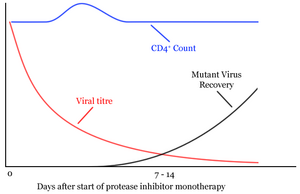
The next clues to the nature of this period of clinical latency came with the first use of antiretroviral protease inhibitor monotherapy [1] (http://www.nature.com/cgi-taf/DynaPage.taf?file=/nature/journal/v373/n6510/abs/373123a0.html&dynoptions=doi1099935896). From the period of clinical latency (a steady state), the viral titre quickly dropped to almost undetectable levels. Also the CD4+ levels quickly increased. Within seven to fourteen days however, levels of mutant virus resistant to the protease inhibitor increased dramatically and the CD4 levels returned to their previous levels at clinical latency. Assuming that the protease inhibitor was 100% effective, the rate of fall of HIV levels equals the rate of HIV production during the steady state or clinical latency period. The rate of HIV production during this period was found to be 108 - 109 virions per day, 30% of the total virus.
Similarly the CD4+ cell turnover was found to be 2x109 cells per day. This implies a constant dynamic struggle between the virus and the host, and raises the possibility that the end of the period of clinical latency is due to the host becoming drained and not able to produce any more CD4+ cells.
The rapid drug resistant variant problem was particularly disheartening for researchers at the time. Although HIV has a high copy error rate (making the creation of drug resistant mutants more likely) it is not significantly higher than other known viruses which do not create mutants as rapidly. HIV is able to so quickly create drug resistant mutants due to its high error rate combined with its enormous virion turnover rate. This highlights the importance of antiretroviral combination therapy over standard monotherapy.
CD4+ cell depletion controversy
After further research, part of the rise in the CD4+ cell levels in the above graph appeared to be due to a redistribution of lymphoid tissue and blood. A study by Hellerstein [2] (http://www.nature.com/cgi-taf/DynaPage.taf?file=/nm/journal/v5/n1/full/nm0199_83.html) used deutero glucose pulse labelling to show that CD4+ cell survival rate in HIV infected hosts decreased by a third, but there was no effect on the rate of production. This implied that CD4+ loss during HIV infection is due to both a shortened survival time of CD4+ cells and a failure to increase levels of CD4+ production.
Another review by Douek [3] (http://arjournals.annualreviews.org/doi/full/10.1146/annurev.immunol.21.120601.141053;jsessionid=iPdXeA4yjvja) looked at the level of thymic re-arrangement excision circles (TRECs) in HIV infected hosts. TRECs are markers for recent thymic emigrants, and their level was found to be substantially reduced in HIV infected hosts. This, along with the recovery of thymic function upon HAART treatment implies that HIV markedly impairs thymic function.
Pathogenesis
HIV causes disease by infecting the CD4+ T cells. These are a subset of leukocytes (white blood cells) that normally coordinate the immune response to infection. By using CD4+ T cells to replicate itself, HIV spreads throughout the body and at the same time depletes the very cells that the body needs to fight the virus. Once an HIV-positive individual's CD4+ T cell count has decreased to a certain threshold, they are prone to a range of diseases that the body can normally control. These opportunistic infections are usually the cause of death.
There are several reasons why HIV is so hard to fight. First, the virus is an RNA virus, using the reverse transcriptase enzyme to convert its RNA into DNA. This additional process results in a greater chance of mutation than in DNA viruses. Therefore, the virus becomes quickly resistant to therapy. Second, the common notion that HIV is a killer feasting on T cells is not true. If HIV were a killer virus, it would have died out soon because there would be too little time for new infections. In reality, HIV stays in the body for years, infecting people through unsafe sex, blood transfusions and breastfeeding of infants by mothers oblivious to their infection. HIV can survive even when drugs eliminate all detectable virons in the blood (viremia). It integrates itself into the DNA of the host cell and can stay there for years, lying dormant, immune to all kinds of therapy because it is just DNA. When the cell divides and the DNA is copied, the virus is copied too. After years, the virus can become active again, seize the cell's machinery and replicate.
In recent years, the notion that the CD4+ T cells decrease because of direct HIV infection has become doubted as well. The HIV coating protein readily detaches from virus particles. The blood becomes filled with these proteins, which can stick to the CD4+ T cells, gluing them together. In addition, they are recognized by the immune system, causing the immune cells to attack their own CD4+ cells.
Treatment
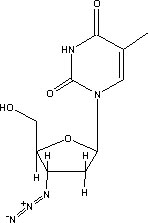
Patients today are given a complex regimen of drugs that attack HIV at various stages in its life cycle. These are known as antiretroviral drugs. They include:
- Protease inhibitors (PIs) inhibit activity of protease, an enzyme used directly by HIV to cleave nascent viral proteins, and so prevent final assembly of HIV virions.
- Reverse
transcriptase inhibitors (RTIs) inhibit the activity of reverse transcriptase,
an enzyme HIV needs to complete infection of a cell. Lack of this enzyme prevents
HIV from building pro-viral DNA based on its RNA. They come in three forms:
- Nonnucleoside reverse transcriptase inhibitors (NNRTIs)
- Nucleoside analog reverse transcriptase inhibitors (NARTIs or NRTIs)
- Nucleotide analog reverse transcriptase inhibitors (NtARTIs or NtRTIs)
- Entry inhibitors inhibit the viral entry into the cell interacting directly with the viral receptor and avoiding the fusion of the viral membrane with the target cell membrane.
Many problems are involved in establishing a course of treatment for HIV. Each effective drug comes with side effects, often serious and sometimes life-threatening in themselves. Common side effects include extreme nausea and diarrhea, liver damage and failure, and jaundice. Any treatment requires regular blood tests to determine continued efficacy (in terms of T-cell count and viral load) and liver function.
Also, no cases are known in which antiviral therapy has been able to terminate HIV infection. More than incremental improvements will be required to change this picture, meaning that HIV-infected people will likely have to stay on treatment life-long. Still, mortality is much lower among people with properly treated HIV infection than among HIV-positive people who got treated either at a late stage of infection or not at all. An important consequence of this is that people with access to adequate healthcare who have acquired HIV are today much better off if they know their status than if they learn about their infection only when symptoms of immune decline appear.
Immunity
About 10% of all Europeans carry a polymorphism of CCR5, a cell surface receptor involved in M-tropic HIV-1 infections. M-tropic HIV-1 uses CCR5 and CD4 receptors to enter target cells, unlike T-tropic HIV which uses CXCR4 with CD4. About 1% of all Europeans are homozygous for this mutation (a 32 base pair deletion), and have a very low risk of HIV-1 infection, although not complete protection.
There is evidence that other individuals may be immune or highly resistant to HIV infection. For instance, studies of prostitutes in sub-Saharan Africa have found some individuals who test negative for HIV despite years of unprotected exposure to hundreds of partners in populations with some of the highest infection rates in the world. This effect, if real, is not yet well understood.
Common misconceptions regarding HIV
- "AIDS
and HIV are the same thing".
- While HIV is the virus that causes AIDS, AIDS refers to the set of immune deficiency symptoms that often do not surface for years after initial infection. Certain types of AIDS defining illnesses must be present for a person to be diagnosed as having AIDS, including a low CD4+ count, typically of 200 or less. HIV itself is a retrovirus that lives in the host before and durring the onset of AIDS.
- "AIDS is not caused by
HIV, but by poverty, anti-HIV drugs etc."
- In AIDS, a number of diseases opportunistically occur. Most of them generally affect in persons with weakened immune systems, but in AIDS, HIV is defined as the ultimate cause. Today, AIDS is diagnosed via the helper T-cell count; in the 1980s unusually severe cases of a combination of opportunistic diseases were considered diagnostic. This obviously resulted in cases in which individuals with symptoms similar to AIDS, but not caused by HIV but by poverty, bad living conditions etc received anti-HIV treatment. (It should be remembered that much of what we 'knew' about HIV until the mid-1990s was misleading and/or even plain wrong). Since the early antiretroviral drugs were highly toxic (most notoriously AZT), this treatment was counterproductive. It should be noted that AIDS was only described in the 1980s, after the start of the HIV epidemic, while similar conditions - wasting, decreased resistance against infections - due to poverty etc. have been around for centuries without raising much interest. Thus, it was the increased occurrence of opportunistic diseases in individuals who would not be expected to suffer from them that alerted doctors that something was wrong. Additionally, what opportunistic diseases occur in AIDS can vary, tuberculosis being more prevalent in Eastern Europe/Russia, hepatitis among IV drug users etc.
- "HIV
only affects homosexuals and drug users".
- Though the risk for infection is indeed statistically greater for gay men and injection drug users, HIV can infect anyone. Babies, women, senior citizens, teenagers, and people of any ethnicity can contract HIV.
- "There is no risk to two
people already infected to have unprotected sex".
- HIV superinfection (or coinfection as it is sometimes called) has thought to be a consequence of unprotected sexual encounters between HIV infected people. Superinfection occurs when a person with HIV gets passed the same strain or a completely new strain, usually as a result of having unprotected sex with another HIV infected person. Superinfection has been demonstrated in laboratory studies as well as in animal trials. For years, proof that it could happen in real life situations has been hard to come by, but recent evidence has surfaced in human case studies that has confirmed that HIV superinfection can occur and can be very problematic for people with HIV.
- "People over age 50 don't
get HIV".
- People over 50 can get HIV. The number of people over age 50 who are newly diagnosed with HIV infection is growing.
- "An
HIV positive woman can't give birth to a healthy baby".
- HIV is sometimes transmitted from mother to unborn child, but not always. The risk is at least 20 to 30% for maternal-fetal transmission of HIV. Delivery via cesarean section and antiretroviral drugs taken during pregnancy can reduce the chance of mother to child infection; Where these treatments are available and the prospective mother is diagnosed early enough, only around two percent of HIV-positive mothers who carry the baby to term will give birth to an infected child. Post-partum infections via breastfeeding are also a problem, especially in the Third World where infant formula may not be available.
- "A
single identifiable person brought HIV to North America"
- See Patient Zero.
Life cycle of HIV
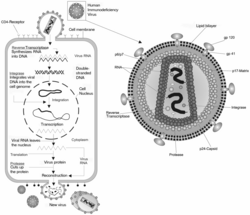
HIV enters a CD4+ helper T-cell by bonding with either CXCR4 or both CXCR4 and CCR5 depending on what stage the HIV infection is in. A cofactor protein (fusin) is required to assist binding of the viron to the membrane of the T-cell. During the early phases of an HIV infection typically both CCR5 and CXCR4 are bound while late stage infection often involve HIV mutations that only bind to CXCR4.
Once HIV has bound to the CD4+ T-cell a viral protein known as GP41 penetrates the cell membrane and the HIV RNA and various enzymes including but not limited to reverse transcriptase, integrase and protease are injected into the cell.
The host T-cell can process RNA into proteins (as in Polio virus) but this doesn't happen with HIV. Instead, HIV is stabilised by copying it into DNA and inserting it into the host cell's chromosomes. This means the virus can perform more subtle functions by using the host transcription machinery. The virus generates DNA from the HIV RNA using the reverse transcriptase enzyme to perform reverse transcription. This process can be inhibited by drugs. If this succeeds the pro-viral DNA must then be integrated into the host cell DNA using the integrase enzyme. If the pro-viral DNA becomes integrated into the host cell's DNA the cell is now fully infected but not actively producing HIV proteins. This is the latent stage of HIV an infection during which the infected cell can be an "unexploded bomb" for potentially a long time.
To actively produce virus, certain transcription factors need to be present in the cell. The most important is called NF-kB (NF Kappa B) and is present once the T cells becomes activated. This means that those cells most likely to be killed by HIV are in fact those currently fighting infection.
The production of the virus is regulated, like many viruses. Initially the integrated provirus is copied to mRNA which is then spliced into smaller chunks. These small chunks produce the regulatory proteins Tat (which encourages new virus production) and Rev. As Rev accumulates it gradually starts to inhibit mRNA splicing. At this stage the structural proteins Gag and Env are produced from the full-length mRNA. Additionally the full-length RNA is actually the virus genome, so it binds to the Gag protein and is packaged into new virus particles.
Interestingly HIV-1 and HIV-2 appear to package their RNA differently - HIV-1 will bind to any appropriate RNA whereas HIV-2 will preferentially bind to the mRNA which was used to create the Gag protein! This may mean that HIV-1 is better able to mutate (HIV-1 causes AIDS faster than HIV-2 and is the majority species of the virus).
The virus starts to form under the cell membrane, in special cholesterol-rich regions, and gradually buds outside. Once outside it has to undergo a maturation step or else it isn't infectious. The virus protease enzyme cleaves Gag into several smaller proteins (Matrix, Capsid, p2, Nucleocapsid, p1 and P6) and this step can be inhibited by drugs. The virus is then able to infect a further cell.
Structure of the virus
HIV looks quite different to the classic retroviruses described in the 1960's. It is around 120nm in diameter (120 billionths of a meter - a red blood cell is around 60 times larger at 7 millionths of a meter) and roughly spherical. There are two forms of the virus. They both consist of a lipid envelope surrounding a protein/RNA core.
Immature form. When the virus leaves the cell it is not infectious and the inner part of the virus particle contains a spherical core (stains dark on electron micrographs). There are also spikes on the outer membrane that are the Env proteins (gp120 and gp41). Sometimes a virus can be seen during the process of budding, when it looks like a dark arc sitting under the cell membrane - this observation meant that HIV was originally classed as a type C retrovirus. The Env proteins link together in groups of three (trimers).
Mature form. Once the virus protease has cleaved the Gag proteins, the core rearranges into a truncated cone (imagine a traffic cone sliced at an angle across the top!) Some reports also show a small filament linking the core to the membrane. The envelope spikes are often much rarer on mature particles since they are easily dislodged. It is the mature conical core that makes HIV so easily identifiable.
Inside the virus there are two identical strands of RNA, in the same way that we have two identical copies of each chromosome. The RNA is coated by the CA protein (formed from Gag) and is not easily seen unless the virus particles are broken apart. The reverse transcriptase enzyme, which includes integrase, is also packaged into the virus along with certain other important proteins (some from the virus, some captured from the cell) and a tRNA molecule that initiates the reverse transcription process. Because the virus contains certain proteins it needs to replicate, injection of the pure RNA will not result in a successful infection.
Transmission of HIV
HIV can be passed through contact with contaminated bodily fluids. This includes blood and semen and any other bodily fluid that may be contaminated with blood. The most common form of transmission of HIV worldwide is sexual contact, whether vaginal or anal. Other significant sources of transmission of HIV has been through sharing of needles or through needlestick injuries (inadvertent injury with a contaminated sharp). HIV can also be spread through blood transfusions in cases where the donors were not screened or inadequately screened for HIV. Patients requiring frequent doses of blood products such as those with hemophilia are at increased risk here.
There is no evidence that HIV can be transmitted through hugging, shaking hands or other simple physical contact where there was no bleeding involved - including contact with carrier's sweat. HIV can theoretically be spread through kissing but the likelihood of this is very, very low unless there is some form of intraoral bleeding involved. There is no evidence that HIV can be spread through vectors such as mosquitoes.
Protection against transmission
Proper use of physical barriers such as the latex condom, have been shown to greatly reduce the risk of transmission of sexually transmitted diseases including HIV.
Reference
- Kahn JO, Walker BD. Acute Human Immunodeficiency Virus type 1 infection. N Engl J Med 1998;331:33-9. PMID 9647878 (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9647878)
- Gilbert et.al. Comparison of HIV-1 and HIV-2 infectivity from a prospective cohort study in Senegal. Stat Med. 2003, Feb 28;22(4):573-93. PMID 12590415 (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12590415)
- Sharp et.al. The origins of acquired immune deficiency syndrome viruses: where and when? Philos Trans R Soc Lond B Biol Sci. 2001 Jun 29;356(1410):867-76. PMID 11405934 (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=11405934)
See also
External links
- Genome (HIV-1) (http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?val=NC_001802)
- Genome (HIV-2) (http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?val=NC_001722)
- "The Molecules of HIV" information resource (http://www.mcld.co.uk/hiv/)
- AIDS/HIV Education (http://www.ericdigests.org/pre-9212/hiv.htm)
- HIV/AIDS Education in Teacher Preparation Programs (http://www.ericdigests.org/1997-3/hiv.html)
- Continuing medical education about HIV for healthcare providers (http://www.cmeonhiv.com)
- How Aids Works (http://health.howstuffworks.com/aids.htm) (with animation)
- CNN.com - New HIV clue may help find vaccine - Februaty 23, 2005 (http://edition.cnn.com/2005/HEALTH/02/23/aids.protein.reut/index.html)